ARLINDA CERGA-PASHOJA1,2, ASMAE DOUKANI1, PARTHENIA GIANNAKOPOULOU2
1. London School of Hygiene and Tropical Medicine, Centre for Global Mental Health, Keppel Street WC1E 7HT; 2. Imperial College London, Neuroepidemiology and Ageing Unit, Charing Cross Campus, St Dunstan’s Road, London, W6 8RP
Corresponding to: Arlinda Cerga-Pashoja, London School of Hygiene and Tropical Medicine, Centre for Global Mental Health, Keppel Street WC1E 7HT, a.cerga-pashoja@imperial.ac.uk,+ 44 2075943332
Care Weekly 2018;2:32-42
Published online November 5, 2018, http://dx.doi.org/10.14283/cw.2018.11
Abstract
Background: Behavioural and Psychological Symptoms of Dementia (BPSD) such as agitation and aggression are core symptoms of dementia and affect almost everyone with the condition. Such symptoms cause distress to the person with dementia and their caregivers and have also been found to predict early institutionalisation as well as death. Historically, BPSD have been managed with medication, typically using anti-psychotic drugs. However, recent data show that anti-psychotic medications increase mortality and the risk of stroke in people with dementia. On the other hand, non-pharmacological interventions such as sensory manipulation, psychological therapies and providing training and psychoeducation for caregivers, present more encouraging results. Consequently, there is a need to explore the potential impact of non-pharmacological interventions on BPSD.
Key words: BPSD, dementia, non-pharmacological treatments.
Introduction
Life expectancy is increasing globally, and is expected to continue to rise, especially in industrialised societies. This is resulting in an ever increasing dementia prevalence world-wide, with 4.6 million new cases being diagnosed each year and a prevalence that is expected to double between 2001 and 2040 (1).
Over 46 million people live with dementia worldwide and this number is estimated to increase to 131.5 million by 2050 (2). Alzheimer’s Society Report (2014) (3) indicates that there are 850,000 people currently living with dementia in the UK. It also claims that 163,000 new cases of dementia occur in England and Wales each year, which means a new case arises every 3.2 minutes. Approximately 6% of people aged over 65 years have some form of dementia (4), with the population prevalence rising to 20% in those aged over 80 years (5).
The Impact of Dementia
The cost of dementia to the economy is immense. The Dementia Report (2014) (3) reveals that dementia costs the UK economy about £26 billion a year, which is more than the cost of cancer and heart disease combined. The global cost is even more prominent being estimated as US $818 billion in 2015, and a trillion dollars by 2018 (6). As a result, dementia has been described as “the greatest medical challenge of the 21st century» (7).
Investments in dementia research, however, remain relatively modest. In the UK, the combined government and charitable investment in dementia research, in 2010 was 12 times lower than spending on cancer research. Over £590 million is spent on cancer research each year, while just £50million is invested in dementia research.
There are 6.5 million people in the UK who provide unpaid care and support to older people with dementia (8). This number is predicted to reach 9 million by 2037 (9). According to the Dementia Report (2010) 25 million people or 42% of the UK population know someone close to them who has been diagnosed with dementia. The Alzheimer’s Research Trust (2010) reports that 1.4 million of carers provide more than 50 hours per week unpaid care, thereby, saving the UK economy £8 billion per year.
Caring for people with dementia comes at a considerable personal financial, physical, mental and psychological price for the carers. Carers of people with dementia experience more physical and mental health problems (10, 11) and get more distressed (12, 13) when compared to their counterparts who look after older people without dementia. Despite the fact that carers bring huge savings to the economy, 75% of them report that they are worse off financially as a result of caregiving (14). Carers report reduced income or working hours, missing out on opportunities for promotion, or being forced to give up work, as a result of their caring responsibilities (15).
Behavioural and Psychological Symptoms of Dementia
Behavioural and psychological symptoms of dementia (BPSD) are also known as neuropsychiatric or non-cognitive symptoms, are common and distressing features of the condition. As highlighted in Table 1, psychological symptoms can include anxiety, depressed mood, hallucinations and delusions. Behavioural symptoms refer to aberrant motor behaviour, verbal and physical aggression, screaming, restlessness, agitation, swearing, wandering, apathy, culturally inappropriate behaviours, disinhibition and hoarding (16). Unlike cognitive functioning of people with dementia that progressively deteriorates, BPSD symptoms typically fluctuate over the course of dementia (17, 18).
Phenomenology, Prevalence and Incidence of BPSD
The term ‘Behavioural and Psychological Symptoms of Dementia’ was introduced in 1999 by the International Psychogeriatric Association (IPA) who has been at the forefront of raising awareness about BPSD.
It is estimated that over 90 percent of people with dementia are likely to experience BPSD such as changes of personality and behaviour (19, 20) and sleep disruption (21). People with dementia are four times more likely to experience BPSD than older adults without dementia (22). Prevalence estimates for BPSD vary due to heterogeneity of population sample studied, in relation to diverse settings, type of dementia, different study designs, study sample size, different instruments used to measure the symptoms and the different definitions used for BPSD (23–27). Such estimates suggest that the prevalence of BPSD is more common in nursing homes than in community settings (28).
The development of BPSD is associated with a worse prognosis and faster rate of dementia progression (29). Untreated BPSD brings about a reduction in the quality of life for the person suffering from the condition and causes significant distress (26, 30, 31). Treating BPSD symptoms, can have a reparative impact on functional impairment, through reducing patient and carer distress and improving quality of life.
BPSD symptoms increase carer stress (32) and have a negative effect on their quality of life (33). Behaviours that challenge carers especially depression and low-mood are reported to have a consistent and powerful negative impact on the psychological health of carers (34). Behavioural and Psychological Symptoms of Dementia cause distress for carers and can contribute to the breakdown in care at home and lead carers to transfer the care of the person with dementia to a care home (35–38). For instance, the increased burden that is placed on the carer in relation to specific BPSD symptoms such as sleep disturbances (i.e. nightly restlessness and wandering) have been found to predict the relocation of care to nursing or residential homes (39. 40).
Untreated BPSD can also cause stress to nursing staff in residential facilities (41, 42) and increase financial costs (30, 43, 44).
Aetiology of BPSD
Different theoretical frameworks have been proposed to explain the aetiology of BPSD. These theories are divided into two major groups, neurobiological and psychosocial, and have driven the development of a variety of distinct interventions for BPSD.
The Neurobiological Theories of BPSD
The neurobiological/genetic theories of BPSD are predominant within medicinal research and practice, and assert that BPSD are the direct cause of cognitive decline, brain dysfunction, imbalance of neurotransmitters and genetic characteristics (45). Boyle and Malloy (2004) claim that apathy is triggered by an interaction between cholinergic deficiency and neuropathological changes in frontal brain regions. Agitation and psychosis are reported to be specifically correlated with a high burden of neurofibrillary tangles (47, 48).
The Neurobiological Theories of BPSD
Cohen-Mansfield (2000) (49) has proposed three psychosocial models of BPSD:
• The unmet needs model
• Learning/behavioural model
• Environmental vulnerability/reduced stress-threshold model
The Unmet Needs model suggests that the dementia process affects both the ability of the person with dementia to use the environment appropriately to meet their needs and their ability to communicate their needs effectively (105–107). The interaction of such impairments with longstanding habits, personality, physical and mental states and unfavourable environmental conditions, can give rise to BPSD.
The Learning/Behavioural model proposes that BPSD develop as the direct result of conditioning. The basis of conditioning suggests that a reward which follows a particular response, acts as a reinforcer and increases the likelihood that the response will be repeated. For example: under-stimulation in a nursing home (bored, lonely) -> triggers agitation in a person with dementia -> staff engage more and try to calm and pacify the resident. While this may fulfil the social need of the person with dementia, the attention received by care staff reinforces agitated behaviours.
Lastly, the Environmental Vulnerability/Reduced Stress-Threshold model suggests that a person’s needs and abilities need to match the environmental demands (105). This model maintains the decreased level of competence of people with dementia, is likely to increase the likelihood that they will be disturbed by the environment. Therefore, environmental stimuli that are inappropriate and exceed the individual’s threshold for tolerating stress, result in negative mood and challenging behaviours (BPSD).
The three psychosocial models described above may be complementary and are not mutually exclusive. Different models may also account for different BPSD symptoms in different individuals.
Treatments for BPSD
Conventionally, a pharmacological approach has dominated the clinical management of BPSD. Common forms of treatments include mood stabilisers, anxiolytics, hypnotic and antipsychotic medications and more recently, cholinesterase inhibitors. Antipsychotic medications (e.g. haloperidol, risperidone, olanzapine) have been overly used to manage behavioural problems such as agitation and aggression. However, there are long-standing concerns that treating aggressive behaviour with pharmacological methods suppresses behaviour without addressing the cause (50, 51).
Use of antipsychotics in dementia increases the likelihood of stroke and premature death, leading health authorities in the United States (52) and the UK (53) to issue caution to clinicians, recommending that the use of such drugs in the care for dementia should be avoided (51, 54). In the UK, the NICE/SCIE guidelines encourage clinicians to treat BPSD with non-pharmacological methods in the first instance, unless the patient is severely distressed and there is an immediate risk of harm to themselves or others (55). Long-term psychosocial strategies may be a better way to achieve the best quality care possible for people with dementia and those whom support them. For this reason, non-pharmacological alternatives are now being considered as first-line management of BPSD (56).
Non-pharmacological interventions for BPSD
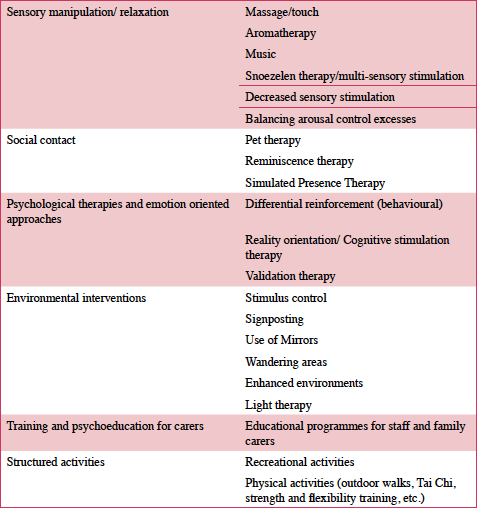
The growing recognition of the importance of BPSD symptoms and the rising concern regarding their treatment with antipsychotic drugs have triggered a surge of interest in developing and applying non-pharmacological interventions as treatments for BPSD. Table 2 presents our summary of non-pharmacological interventions for BPSD. The main headings represent the category under which individual interventions have been included. Most of the individual therapies may be categorised under more than one heading, for example reduced sensory stimulation can be classified as sensory manipulation as well as environment manipulation and social contact. However, for ease of description individual interventions have been categorised under one heading.
Sensory manipulation treatments are primarily used with people with moderate to severe dementia and have been focusing on both stimulation enhancement and reduction. Stimulation enhancement approaches have been used to provide meaningful stimulation, reduce anxiety/agitation and improve mood and are based on the ‘unmet needs’ and ‘environmental vulnerability’ models, which propose that BPSD may result from periods of sensory deprivation (57). Massage therapies have included hand massage with essential oils (58–60), electrical nerve stimulation (61) and craniosacral therapy1 (62). All these studies, apart from Scherder et al. (1995) have indicated decrease on agitation, wandering and fidgety behaviours, however, they are quite small with samples varying from 14-30 participants and the methodologies used are not robust (not blinded, no control group) (63).
• Aromatherapy involves the use of essential oils such as lavender, thyme and melissa (lemon) balm for treatment of agitation in people with dementia. These oils have been applied in different ways such as by diffusion in communal areas (64), bedside diffusers (65), applied to the skin (66) and as sachets (67). Most of these studies have reported significant reduction in agitation and excellent compliance with the intervention (68). The study from Ballard and colleagues (2002) is a randomised controlled trial (RCT) that reported not only significant reductions in agitation but also excellent compliance with the intervention. However, the intervention itself was more than just aromatherapy as it also involved increased social contact whereby Melissa balm was applied twice a day on participants’ faces and arms, which may also be considered as massage. Therefore, the benefit of aromatherapy is still under investigation and it has been reported as one of the fastest growing complementary therapies (69, 70).
• Music interventions have been quite heterogeneous, ranging from listening to music for relaxation and anxiety reduction (71, 72), during mealtimes (73, 74) or bath-times (75), to dancing, singing, playing musical instruments, or participating in composition and improvisation sessions (57, 76, 77). All of the above studies reported some benefits in terms of decreased agitation, reduction of aggressive behaviours and grater positive engagement. The main disadvantage of music intervention studies is that most of them have very small sample sizes (ranging from 10-46 participants); have employed within-participants experimental design; and use direct observational methods which are susceptible to rater bias and Hawthorne effect. Therefore the chance of obtaining significant results is reduced and the findings remain inconclusive (78). White noise (environmental sounds) has also been used to induce relaxation and sleep, and consequently reduce verbal agitation and wandering (79). Different music interventions feed into different psychosocial theories. For example listening for relaxation is based on the unmet needs and environmental vulnerability hypotheses; music use during bath and mealtimes is based on the above mentioned hypotheses as well as the learning/behavioural theory.
• Multi-sensory stimulation (MSS) or Snoezelen therapy, which is also considered as an emotion-oriented approach, combines relaxation and exploration of sensory stimuli such as lights (e.g. fiber optics), sounds and tactile sensations (cushions, vibration pads). This therapy is usually delivered in dedicated rooms and sessions are tailored to individual needs (68). Multi-sensory stimulation is primarily used to reduce apathy in people with advanced dementia. Baker et al. (2001) examined the effects of MSS on 25 participants with moderate to severe dementia through an RCT and found significant reduction in dysphoric mood. However, when the same authors (81) ran a second, bigger trial (n=65) they did not find any significant differences between the control and intervention arms. The cost and complexity of MSS rooms can be a barrier to using them especially as evidence in their favour is not very robust.
• Decreased sensory stimulation interventions are based on the ‘reduced stress threshold’ model, which suggests that BPSD can result as a consequence of over or inappropriate stimulation. This approach has been applied in care or residential homes where the levels of over-stimulation are higher than family homes. Two small studies (82, 83), with samples of 11 participants each, explored benefits of “quiet” interventions in agitation symptoms. Meyer et al. (1992) introduced a ‘quiet week’ which included turning off the television, lowering voices and reducing fast movement by staff at a day centre, and found a significant decrease in agitated behaviours. Cleary et al. (1988) took a more holistic approach by removing sources of stimulation such as TVs, radios and telephones as well as by manipulating the environment (introducing: smaller tables for eating and activities or neutrally painted walls), educating carers and training staff. These authors also reported decrease in agitation. Overall, there is limited evidence about the effectiveness of these interventions on BPSD.
Kovach et al. (2004) has taken a noteworthy approach to the subject of stimulation and its relationship to BPSD. These authors propose a model called Kovach’s Model of Imbalance of Sensoristasis, which describes the importance of keeping in balance sensory-stimulating and sensory-calming activities as this imbalance can give rise to or exacerbate agitation in advanced dementia. This model is consistent with Cohen-Mansfield’s (2000) environmental vulnerability/reduced stress-threshold model.
Social Contact
The next group of interventions include interventions that focus on real or stimulated social contacts and are based on the ‘unmet needs’ model of BPSD. Several studies suggest beneficial effect of pet therapy on agitation and verbal aggression (85–88).
• Reminiscence therapy (RT) uses materials (e.g. old newspapers, photographs and household items), music and art to stimulate memories and enable people to relive past experiences that are highly significant to them. This approach was originally but unsuccessfully utilised as an intervention for improving cognitive symptoms of dementia, but it has been shown to improve level of psycho-social wellbeing of people with dementia (Brooker & Duce, 2000; Lai, Chi, & Kayser-Jones, 2004; Woods, Spector, Jones, Orrell, & Davies, 2005). Reminiscence therapy is flexible and can be adapted to groups as well as individual needs. However, there is limited evidence of a significant impact of RT in BPSD (Woods et al., 2005).
• Simulated presence therapy is grounded in the unmet needs and environmental vulnerability theories and entails use of videos or audio recordings of family members sharing conversations and memories with the person with dementia. This intervention has been used in care and residential homes. Two studies have reported reduction in physical and verbal agitation and greater frequency of happy expressions during treatment, however, these benefits do not seem to last beyond exposure time (92, 93).
Psychological therapies and emotion oriented approaches
• Differential reinforcement aims to reduce or eliminate maladaptive behaviours by using positive reinforcement in a structured way to increase desirable behaviour and is based on the ‘learning/behavioural model’. Rogers et al. (1999) applied behavioural rehabilitation to reduce disruptive behaviour in nursing home residents with dementia. Doyle and colleagues (1997) also used reinforcement of quiet behaviour and environmental stimulation to decrease noise-making in 12 long-term-care residents with severe dementia. There have not been any reports of more recent interventions of differential reinforcement.
• Reality orientation (RO) is based on the idea that impairment in orientation and confusion prevent people with dementia from functioning well and is supported by the environmental vulnerability and the unmet needs models. RO facilitates re-orientation through: reminding people with dementia of facts about themselves; and through environmental manipulation (such as using signposting, clocks, calendars, newspapers, television, pictures, personal belongings). The efficacy of RO has been criticised by several authors who found RO classes non-efficacious (96); with little long-term effect (97); or argued that RO can have a negative effect on mood through reminding people of their prognosis (98, 99). Dietch and colleagues (1989) claimed that insensitive use of RO through incessantly correcting and challenging people with dementia could result on demeaning and confrontational experiences for this vulnerable population. However, more recent reviews (Spector, Davies, Woods, & Orrell, 2000; Spector, Orrell, Davies, & Woods, 2001) have been quite favourable to RO and its outcomes. In fact it seems as though, after a loss of interest for this approach in the nineteen eighties, interest in RO has been reawakened (Spector et al., 2001; Woods, 2002) under the new term Cognitive stimulation therapy (CST).
• Cognitive stimulation therapy, unlike RO, is grounded in person-centred care (104) and its key principles are to appropriately and sensitively use multi-sensory stimulation to re-orient people with dementia and strengthen relationships with carers (Spector, Orrell, & Goyder, 2013). This approach is predominantly used in group of patients with mild to moderate dementia, as participants need to be able to carry out meaningful conversations and participate in group activities.
• Validation therapy (VT) is based on the Rogerian humanistic psychology argument that BPSD symptoms are strategies used by people with dementia to avoid stress, boredom and the painful reality of their condition (97). Validation therapists propose that empathic communication with individuals with dementia is essential rather than their orientation to the present. Neal and Barton Wright (2003) evaluated validation therapy through a Cochrane review and concluded that evidence about the efficacy of validation therapy is insufficient.
Environmental interventions
Environmental interventions include modifications of the factors that may cause or exacerbate BPSD such as: excessive noise (reduced stress threshold model), lack of routine (unmet needs model), inadequate lighting (environmental vulnerability model), confusing surroundings, and excessive demands by staff in residential settings (learning/behavioural model) (107). Many of these approaches have focussed exclusively on assisted-living facilities. Some of the environmental interventions include:
• Enhanced environments – Several non-randomised trials have investigated the effect of environment manipulation on agitation symptoms and exit-seeking behaviours of people with dementia in residential settings. Different approaches entailed: painting two dimensional grids on the floor in front of exit doors (108–110); painting murals over doorways (111); and placing blinds and cloth barriers over doors or door handles (112, 113). Chafetz (1990) concluded that the two-dimensional grid is ineffective, however, the other authors reported on reduced exiting behaviours and ambulation.
• Wandering areas/ Removal of restraints – A few authors have criticised the reduced autonomy in institutionalised patients, and have argued that these settings exacerbate or even cause BPSD. Two studies found that unlocking exit doors in a residential home, and release from mandatory confinement in an acute unit reduced agitation as well as both physical and verbal aggression (114, 115).
• Light therapy – Light therapy has been utilised to improve circadian rhythms, which are impaired in people with dementia (116). This approach is supported by the ‘unmet needs’ model as it attempts to improve sleep (117, 118) and consequently reduce agitation (119, 120). The above studies have reported benefits on sleep and agitation, but other studies have reported no effect (121, 122). Overall, the support for this approach remains inconclusive as the reported studies are small non-RCTs (57, 123, 124).
Training and psychoeducation programmes for carers
This approach focuses on improving carers’ knowledge of dementia and BPSD, improving communication with people with dementia, and on providing potential management strategies for BPSD. Reduced agitation has been reported after training staff on communication skills (125), empathy (126), tailored and focused care (127–129). Psychoeducation interventions with family carers have also resulted in improved mood (130) and decreased or delayed institutionalisation (131, 132). All the above studies report improved outcomes immediately after the intervention, however this effect ceases not long afterwards, indicating that educational programmes should be of an ongoing nature rather than a one-off intervention (133).
Structured activities
• Recreational activities such as sewing, dancing, games, playing instruments have been reported to have a positive effect on agitation (134) and wandering behaviours (135, 136). These findings, however, are based on a small number of non-randomised studies.
• Physical activities include various types of physical exercises such as outdoor walks (137, 138), Tai Chi (139), strength and flexibility training (140), walking, cycling, chair based exercise (141).
Conclusions
Behavioural and psychological symptoms are some of the most common elements and signs of dementia. Their heterogenous aetiology is drawn from both neurobiological and psychosocial factors as well as environmental ones. Pharmacological approaches have dominated BPSD treatment plans during the past years, although concerns regarding the long-term impact on disease and mortality overall along with their role in targeting the actual causes rather than the symptoms themselves have frequently been raised. As such, most recently, alternative approaches, utilizing non-pharmacological interventions have gained increased attention with positive results. Sensory manipulation interventions, such as aromatherapy and decreased sensory stimulation, psychological therapies and emotion-oriented approaches, as well as training in communication and psychoeducation of the caregivers, present the most encouraging results. In addition, and in the same context, environmental interventions underline the importance of light therapy and enhanced environments approaches. Consequently, non-pharmacological interventions could potentially play a pivotal in BPSD management. They offer an individualized treatment plan free of pharmacological side effects while facilitating a strong interaction between people suffering from dementia and their caregivers with significant long-term impact. Despite the encouraging evidence, non-pharmacological interventions are still quite limited. Therefore, further research would be necessary to explore the full potential of those interventions on BPSD.
Conflict of Interest
We declare that we have no conflict of interest.
References
1. Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet [Internet]. 2005 Dec 17 [cited 2014 May 29];366(9503):2112–7.
2. Prince M, Wimo A, Guerchet M, Gemma-Claire Ali M, Wu Y-T, Prina M, et al. World Alzheimer Report 2015 The Global Impact of Dementia An AnAlysIs of prevAlence, IncIDence, cosT AnD TrenDs. 2015 [cited 2018 Jun 13];
3. Alzheimer’s Society. Dementia 2014: Opportunity for change [Internet]. London; 2014 [cited 2014 Nov 18].
4. Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology [Internet]. 2000 Jan [cited 2014 Jun 17];54(11 Suppl 5):S4-9.
5. Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord [Internet]. 2012 Jan [cited 2015 Dec 29];26(4):335–43.
6. Prince M, Wimo A, Guerchet M, Gemma-Claire Ali M, Wu Y-T, Prina M, et al. World Alzheimer Report 2015 The Global Impact of Dementia An AnAlysIs of prevAlence, IncIDence, cosT AnD TrenDs. 2015;
7. Alzheimer’s Research Trust. DEMENTIA 2010 The economic burden of dementia and associated research funding in the United Kingdom. 2010.
8. Office of National Statistics. Census – unpaid care snapshot. 2011.
9. Buckner L, Yeandle S. Valuing Carers 2011 Calculating the value of carers’ support. 2011.
10. Moise P, Schwarzinger M. Dementia care in 9 OECD countries: a comparative analysis OECD Health Working Paper no. 13. Paris; 2004.
11. Pinquart M, Sörensen S. Correlates of physical health of informal caregivers: a meta-analysis. J Gerontol B Psychol Sci Soc Sci [Internet]. 2007 Mar [cited 2015 Jan 14];62(2):P126-37.
12. The Princess Royal Trust for Carers 2012. Always On Call , Always Concerned: A Survey of the Experiences of Older Carers. 2011.
13. Schulz R, Martire LM. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry [Internet]. 2004 [cited 2015 Jan 14];12(3):240–9.
14. Carers UK. Carers in crisis. 2008.
15. Carers Week. Prepared to Care? Exploring the impact of caring on people’s lives. 2013.
16. Desai AK, Grossberg GT. Recognition and Management of Behavioral Disturbances in Dementia. Prim Care Companion J Clin Psychiatry [Internet]. 2001 Jun [cited 2014 Jul 7];3(3):93–109.
17. Ballard C, Howard R. Neuroleptic drugs in dementia: benefits and harm. Nat Rev Neurosci [Internet]. 2006 Jun [cited 2015 Jan 13];7(6):492–500. 18. Lawlor BA. Behavioral and psychological symptoms in dementia: the role of atypical antipsychotics. J Clin Psychiatry [Internet]. 2004 Jan [cited 2015 Jan 22];65 Suppl 1:5–10.
19. Aalten P, de Vugt ME, Lousberg R, Korten E, Jaspers N, Senden B, et al. Behavioral problems in dementia: a factor analysis of the neuropsychiatric inventory. Dement Geriatr Cogn Disord [Internet]. 2003 [cited 2014 Jul 7];15(2):99–105.
20. Overshott R, Byrne J, Burns A. Nonpharmacological and pharmacological interventions for symptoms in Alzheimer’s disease. Expert Rev Neurother [Internet]. 2004 Sep [cited 2014 Jul 7];4(5):809–21.
21. Boeve BF, Silber MH, Ferman TJ. Current management of sleep disturbances in dementia. Curr Neurol Neurosci Rep [Internet]. 2002 Mar [cited 2014 Jul 7];2(2):169–77.
22. Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry [Internet]. 2000 May [cited 2015 Jan 26];157(5):708–14.
23. Aalten P, Verhey FRJ, Boziki M, Bullock R, Byrne EJ, Camus V, et al. Neuropsychiatric syndromes in dementia: Results from the European Alzheimer disease Consortium: Part I. Dement Geriatr Cogn Disord. 2007;24:457–63.
24. Savva GM, Zaccai J, Matthews FE, Davidson JE, McKeith I, Brayne C. Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. Br J Psychiatry. 2009;194(3):212–9.
25. Lyketsos CG, Lopez O, Jones B, Fitzpatrick A, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairement: results from the cardiovascular health study. JAMA. 2002;(288):1475–83.
26. Finkel SI, Costa e Silva J, Cohen G, Miller S, Sartorius N. Behavioral and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Int Psychogeriatr [Internet]. 1996 Jan [cited 2014 Jul 8];8 Suppl 3:497–500.
27. Ikeda M, Fukuhara R, Shigenobu K, Hokoishi K, Maki N, Nebu A, et al. Dementia associated mental and behavioural disturbances in elderly people in the community: findings from the first Nakayama study. J Neurol Neurosurg Psychiatry [Internet]. 2004 Jan [cited 2015 Jan 26];75(1):146–8.
28. Australian Medicines Handbook. Australian Medicines Handbook Drug Choice Companion: Aged Care. 2nd ed. Adelaide: Australian Medicines Handbook; 2006.
29. Paulsen JS, Salmon DP, Thal LJ, Romero R, Weisstein-Jenkins C, Galasko D, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology [Internet]. 2000 May 23 [cited 2014 Nov 18];54(10):1965–71.
30. Cohen-Mansfield J. Assessment of disruptive behavior/agitation in the elderly: function, methods, and difficulties. J Geriatr Psychiatry Neurol [Internet]. 1995 Jan [cited 2014 Jul 7];8(1):52–60.
31. Chan AP. Alzheimer’s Disease Research Trends [Internet]. Nova Publishers; 2007 [cited 2015 Oct 5]. 309 p.
32. Ornstein K, Gaugler JE. The problem with “problem behaviors”: a systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient-caregiver dyad. Int Psychogeriatr [Internet]. Cambridge University Press; 2012 Oct 1 [cited 2015 Aug 24];24(10):1536–52. A
33. Ballard C, Lowery K, Powell I, OBrien J, James I. Impact of Behavioral and Psychological Symptoms of Dementia on Caregivers. Int psychogeriatrics [Internet]. Cambridge University Press; 2000 Jul 1 [cited 2015 Sep 3];12(S1):93–105.
34. Hooker K, Bowman SR, Coehlo DP, Lim SR, Kaye J, Guariglia R, et al. Behavioral Change in Persons With Dementia: Relationships With Mental and Physical Health of Caregivers. Journals Gerontol Ser B Psychol Sci Soc Sci [Internet]. 2002 Sep 1 [cited 2015 Aug 13];57(5):P453–60. 2075943332
35. Banerjee S. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg psychiatry [Internet]. 2003 Sep 1 [cited 2014 Jul 8];74(9):1315–6.
36. Donaldson C, Tarrier N, Burns A. Determinants of carer stress in Alzheimer’s disease. Int J Geriatr Psychiatry [Internet]. 1998 Apr [cited 2014 Jul 7];13(4):248–56.
37. Finkel S. Introduction to behavioural and psychological symptoms of dementia (BPSD). International journal of geriatric psychiatry. 2000.
38. Lawlor B. Managing behavioural and psychological symptoms in dementia. Br J Psychiatry. 2002;181(6).
39. Schur D, Whitlatch CJ. Circumstances leading to placement: a difficult caregiving decision. Lippincotts Case Manag [Internet]. 2003 [cited 2014 Jul 7];8(5):187-95; quiz 196-7.
40. Lindsay J, Anderson L. Dementia / Alzheimer’s Disease. BMC Womens Health [Internet]. 2004 Aug 25 [cited 2014 Jul 7];4 Suppl 1:S20.
41. Rodney V. Nurse stress associated with aggression in people with dementia: its relationship to hardiness, cognitive appraisal and coping. J Adv Nurs [Internet]. 2000 Jan [cited 2014 Jul 7];31(1):172–80.
42. Draper B, Snowdon J, Meares S, Al E. Case-controlled study of nursing home residents referred for treatment of vocally disruptive behavior. Int Psychogeriatrics. 2000;12:333–4.
43. Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess [Internet]. 2005 Mar [cited 2014 Jun 12];9(10):1–93.
44. O’Donnell BF, Drachman DA, Barnes HJ, Peterson KE, Swearer JM, Lew RA. Incontinence and Troublesome Behaviors Predict Institutionalization in Dementia. J Geriatr Psychiatry Neurol [Internet]. 1992 Jan 1 [cited 2014 Jul 8];5(1):45–52.
45. Kar N. Behavioral and psychological symptoms of dementia and their management. Indian J Psychiatry [Internet]. Medknow Publications; 2009 Jan 1 [cited 2014 May 28];51 Suppl 1(Suppl1):S77-86.
46. Boyle PA, Malloy PF. Treating apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord [Internet]. 2004 Jan [cited 2015 Jun 2];17(1–2):91–9.
47. Tekin S, Mega MS, Masterman DM, Chow T, Garakian J, Vinters H V, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol [Internet]. 2001 Mar [cited 2015 Jun 2];49(3):355–61.
48. Barber R, Snowden JS, Craufurd D. Frontotemporal dementia and Alzheimer’s disease: retrospective differentiation using information from informants. J Neurol Neurosurg Psychiatry [Internet]. 1995 Jul [cited 2015 Jun 2];59(1):61–70.
49. Cohen-Mansfield J. Theoretical Frameworks for Behavioral Problems in Dementia. Alzheimers Care Q. 2000;1(4).
50. Ballard C, Corbett A, Chitramohan R, Aarsland D. Management of agitation and aggression associated with Alzheimer’s disease: controversies and possible solutions. Curr Opin Psychiatry [Internet]. 2009 Nov [cited 2015 Jun 12];22(6):532–40.
51. Banerjee S. The use of antipsychotic medication for people with dementia: Time for action. Dep Heal [Internet]. 2009 [cited 2014 Jul 7];
52. Food and Drugs Administration. Information on Conventional Antipsychotics [Internet]. FDA. 2011.
53. National Institute for Health and Clinical Excellence. Supporting people with dementia and their carers in health and social care. London; 2006.
54. Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, et al. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol [Internet]. Elsevier Ltd; 2009 Feb [cited 2012 Nov 16];8(2):151–7.
55. Clark DM. Implementing NICE guidelines for the psychological treatment of depression and anxiety disorders: the IAPT experience. Int Rev Psychiatry [Internet]. 2011 Aug [cited 2014 Oct 26];23(4):318–27.
56. Hersch EC, Falzgraf S. Management of the behavioral and psychological symptoms of dementia. Clin Interv Aging [Internet]. 2007 Jan [cited 2014 Jul 7];2(4):611–21.
57. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. Am J Geriatr Psychiatry [Internet]. 2001 Jan [cited 2015 Apr 13];9(4):361–81.
58. Kilstoff K, Chenoweth L. New approaches to health and well-being for dementia day-care clients, family carers and day-care staff. Int J Nurs Pract [Internet]. 1998 Jun [cited 2015 May 27];4(2):70–83.
59. Rowe M, Alfred D. The effectiveness of slow-stroke massage in diffusing agitated behaviors in individuals with Alzheimer’s disease. J Gerontol Nurs [Internet]. 1999 Jun [cited 2015 May 27];25(6):22–34.
60. Kim EJ, Buschmann MT. The effect of expressive physical touch on patients with dementia. Int J Nurs Stud [Internet]. 1999 Jun [cited 2015 May 27];36(3):235–43.
61. Scherder EJA, Bouma A, Steen AM. Effects of short-term transcutaneous electrical nerve stimulation on memory and affective behaviour in patients with probable Alzheimer’s disease. Behav Brain Res [Internet]. 1995 Mar [cited 2015 May 27];67(2):211–9.
62. Gerdner LA, Hart LK, Zimmerman MB. Craniosacral still point technique: exploring its effects in individuals with dementia. J Gerontol Nurs [Internet]. 2008 Mar [cited 2015 May 27];34(3):36–45.
63. Kverno KS, Black BS, Nolan MT, Rabins P V. Research on treating neuropsychiatric symptoms of advanced dementia with non-pharmacological strategies, 1998-2008: a systematic literature review. Int Psychogeriatr [Internet]. 2009 Oct [cited 2015 Jan 13];21(5):825–43.
64. Holmes C, Hopkins V, Hensford C, MacLaughlin V, Wilkinson D, Rosenvinge H. Lavender oil as a treatment for agitated behaviour in severe dementia: a placebo controlled study. Int J Geriatr Psychiatry [Internet]. 2002 Apr [cited 2015 May 27];17(4):305–8.
65. Lin PW, Chan W, Ng BF, Lam LC. Efficacy of aromatherapy (Lavandula angustifolia) as an intervention for agitated behaviours in Chinese older persons with dementia: a cross-over randomized trial. Int J Geriatr Psychiatry [Internet]. 2007 May [cited 2015 May 27];22(5):405–10.
66. Ballard CG, O’Brien JT, Reichelt K, Perry EK. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with Melissa. J Clin Psychiatry [Internet]. 2002 Jul [cited 2015 May 27];63(7):553–8.
67. Snow LA, Hovanec L, Brandt J. A controlled trial of aromatherapy for agitation in nursing home patients with dementia. J Altern Complement Med [Internet]. 2004 Jun [cited 2015 May 27];10(3):431–7.
68. Douglas S. Non-pharmacological interventions in dementia. Adv Psychiatr Treat [Internet]. 2004 May 1 [cited 2015 Feb 10];10(3):171–7.
69. Burns A. Sensory stimulation in dementia. BMJ [Internet]. 2002 Dec 7 [cited 2015 Jun 11];325(7376):1312–3.
70. Forrester LT, Maayan N, Orrell M, Spector AE, Buchan LD, Soares-Weiser K. Aromatherapy for dementia. Cochrane database Syst Rev [Internet]. 2014 Jan [cited 2015 Sep 2];2:CD003150.
71. Holmes C, Knights A, Dean C, Hodkinson S, Hopkins V. Keep music live: music and the alleviation of apathy in dementia subjects. Int Psychogeriatr [Internet]. 2006 Dec [cited 2015 May 20];18(4):623–30.
72. Sung H-C, Chang AM, Lee W-L. A preferred music listening intervention to reduce anxiety in older adults with dementia in nursing homes. J Clin Nurs [Internet]. 2010 Apr [cited 2015 Aug 4];19(7–8):1056–64.
73. Chang F-Y, Huang H-C, Lin K-C, Lin L-C. The effect of a music programme during lunchtime on the problem behaviour of the older residents with dementia at an institution in Taiwan. J Clin Nurs [Internet]. 2010 Apr [cited 2015 Aug 4];19(7–8):939–48.
74. Hicks-Moore SL. Relaxing music at mealtime in nursing homes: effects on agitated patients with dementia. J Gerontol Nurs [Internet]. 2005 Dec [cited 2015 Aug 5];31(12):26–32.
75. Clark ME, Lipe AW, Bilbrey M. Use of music to decrease aggressive behaviors in people with dementia. J Gerontol Nurs [Internet]. 1998 Jul [cited 2015 May 27];24(7):10–7.
76. Sherratt K, Thornton A, Hatton C. Music interventions for people with dementia: a review of the literature. Aging Ment Health [Internet]. 2004 Jan [cited 2015 Sep 4];8(1):3–12.
77. Ledger AJ, Baker FA. An investigation of long-term effects of group music therapy on agitation levels of people with Alzheimer’s Disease. Aging Ment Health [Internet]. 2007 May [cited 2015 Aug 5];11(3):330–8.
78. Vasionytė I, Madison G. Musical intervention for patients with dementia: a meta-analysis. J Clin Nurs [Internet]. 2013 May [cited 2015 Mar 16];22(9–10):1203–16.
79. Burgio L, Scilley K, Hardin JM, Hsu C, Yancey J. Environmental “white noise”: an intervention for verbally agitated nursing home residents. J Gerontol B Psychol Sci Soc Sci [Internet]. 1996 Nov [cited 2015 May 28];51(6):P364-73.
80. Baker R, Bell S, Baker E, Gibson S, Holloway J, Pearce R, et al. A randomized controlled trial of the effects of multi-sensory stimulation (MSS) for people with dementia. Br J Clin Psychol [Internet]. 2001 Mar [cited 2015 May 27];40(Pt 1):81–96.
81. Baker R, Holloway J, Holtkamp CCM, Larsson A, Hartman LC, Pearce R, et al. Effects of multi-sensory stimulation for people with dementia. J Adv Nurs [Internet]. 2003 Sep [cited 2015 May 27];43(5):465–77.
82. Meyer D, Dorbacker B, O’Rourke J. Effects of a “quiet week” intervention on behavior in an Alzheimer boarding home. Am J Alzheimer’ care Relat Disord Res. 1992;6:2–6.
83. Cleary TA, Clamon C, Price M, Shullaw G. A reduced stimulation unit: effects on patients with Alzheimer’s disease and related disorders. Gerontologist [Internet]. 1988 Aug [cited 2015 May 27];28(4):511–4.
84. Kovach CR, Taneli Y, Dohearty P, Schlidt AM, Cashin S, Silva-Smith AL. Effect of the BACE intervention on agitation of people with dementia. Gerontologist [Internet]. 2004 Dec [cited 2015 May 27];44(6):797–806.
85. Churchill M, Safaoui J, McCabe BW, Baun MM. Using a therapy dog to alleviate the agitation and desocialization of people with Alzheimer’s disease. J Psychosoc Nurs Ment Health Serv [Internet]. 1999 Apr [cited 2015 May 27];37(4):16–22.
86. Fritz CL, Farver TB, Kass PH, Hart LA. Association with companion animals and the expression of noncognitive symptoms in Alzheimer’s patients. J Nerv Ment Dis [Internet]. 1995 Jul [cited 2015 May 27];183(7):459–63.
87. Fritz CL, Farver TB, Hart LA, Kass PH. Companion animals and the psychological health of Alzheimer patients’ caregivers. Psychol Rep [Internet]. 1996 Apr [cited 2015 May 27];78(2):467–81.
88. Zisselman MH, Rovner BW, Shmuely Y, Ferrie P. A pet therapy intervention with geriatric psychiatry inpatients. Am J Occup Ther [Internet]. 1996 Jan [cited 2015 May 27];50(1):47–51.
89. Brooker D, Duce L. Wellbeing and activity in dementia: A comparison of group reminiscence therapy, structured goal-directed group activity and unstructured time. Aging Ment Health [Internet]. Taylor & Francis Group; 2000 Nov 9 [cited 2015 Mar 9];4(4):354–8.
90. Lai CKY, Chi I, Kayser-Jones J. A randomized controlled trial of a specific reminiscence approach to promote the well-being of nursing home residents with dementia. Int Psychogeriatr [Internet]. 2004 Mar [cited 2015 May 27];16(1):33–49.
91. Woods B, Spector A, Jones C, Orrell M, Davies S. Reminiscence therapy for dementia. Cochrane database Syst Rev [Internet]. 2005 Jan [cited 2015 May 27];(2):CD001120.
92. Camberg L, Woods P, Ooi WL, Hurley A, Volicer L, Ashley J, et al. Evaluation of Simulated Presence: a personalized approach to enhance well-being in persons with Alzheimer’s disease. J Am Geriatr Soc [Internet]. 1999 Apr [cited 2015 May 27];47(4):446–52.
93. Garland K, Beer E, Eppingstall B, O’Connor DW. A comparison of two treatments of agitated behavior in nursing home residents with dementia: simulated family presence and preferred music. Am J Geriatr Psychiatry [Internet]. 2007 Jun [cited 2015 May 27];15(6):514–21.
94. Rogers JC, Holm MB, Burgio LD, Granieri E, Hsu C, Hardin JM, et al. Improving morning care routines of nursing home residents with dementia. J Am Geriatr Soc [Internet]. 1999 Sep [cited 2015 May 28];47(9):1049–57.
95. Doyle C, Zapparoni T, O’Connor D, Runci S. Efficacy of psychosocial treatments for noisemaking in severe dementia. Int Psychogeriatr [Internet]. 1997 Dec [cited 2015 May 28];9(4):405–22.
96. Hanley IG, McGuire RJ, Boyd WD. Reality orientation and dementia: a controlled trial of two approaches. Br J Psychiatry [Internet]. 1981 Jan 1 [cited 2015 May 29];138(1):10–4.
97. Hitch S. Cognitive therapy as a tool for caring for the elderly confused person. J Clin Nurs [Internet]. 1994 Jan [cited 2015 May 29];3(1):49–55.
98. Goudie F, Stokes G. Understanding confusion. Nurs Times [Internet]. 1989 Jan [cited 2015 May 29];85(39):35–7.
99. Baines S, Saxby P, Ehlert K. Reality orientation and reminiscence therapy. A controlled cross-over study of elderly confused people. Br J Psychiatry [Internet]. 1987 Aug [cited 2015 May 29];151:222–31.
100. Dietch JT, Hewett LJ, Jones S. Adverse effects of reality orientation. J Am Geriatr Soc [Internet]. 1989 Oct [cited 2015 May 29];37(10):974–6.
101. Spector A, Davies S, Woods B, Orrell M. Reality orientation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Gerontologist [Internet]. 2000 Apr 1 [cited 2015 May 14];40(2):206–12.
102. Spector A, Orrell M, Davies S, Woods B. Can reality orientation be rehabilitated? Development and piloting of an evidence-based programme of cognition-based therapies for people with dementia. Neuropsychol Rehabil [Internet]. Taylor & Francis Group; 2001 Jul 22 [cited 2015 May 29];11(3–4):377–97.
103. Woods B. Reality orientation: a welcome return? Age Ageing [Internet]. 2002 May [cited 2015 May 29];31(3):155–6.
104. Kitwood T. Dementia reconsidered: The person comes first. Buckingham, editor. Open University Press; 1997.
105. Spector A, Orrell M, Goyder J. A systematic review of staff training interventions to reduce the behavioural and psychological symptoms of dementia. Ageing Res Rev [Internet]. 2013 Jan [cited 2015 Mar 2];12(1):354–64.
106. Neal M, Barton Wright P. Validation therapy for dementia. Cochrane database Syst Rev [Internet]. 2003 Jan [cited 2015 Apr 27];(3):CD001394.
107. Gauthier S, Cummings J, Ballard C, Brodaty H, Grossberg G, Robert P, et al. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. 2010 May;22(3):346–72.
108. Hewawasam L. Floor patterns limit wandering of people with Alzheimer’s. Nurs Times [Internet]. 1996 Jan [cited 2015 Jun 1];92(22):41–4.
109. Hussian RA, Brown DC. Use of two-dimensional grid patterns to limit hazardous ambulation in demented patients. J Gerontol [Internet]. 1987 Sep [cited 2015 Jun 1];42(5):558–60.
110. Chafetz PK. Two-dimensional grid is ineffective against demented patients’ exiting through glass doors. Psychol Aging [Internet]. 1990 Mar [cited 2015 Jun 1];5(1):146–7.
111. Kincaid C, Peacock JR. The Effect of a Wall Mural on Decreasing Four Types of Door-Testing Behaviors. J Appl Gerontol [Internet]. 2003 Feb 1 [cited 2015 Jun 1];22(1):76–88.
112. Dickinson JI, McLain-Kark J, Marshall-Baker A. The effects of visual barriers on exiting behavior in a dementia care unit. Gerontologist [Internet]. 1995 Feb [cited 2015 Jun 1];35(1):127–30.
113. Namazi KH, Rosner TT, Calkins MP. Visual barriers to prevent ambulatory Alzheimer’s patients from exiting through an emergency door. Gerontologist [Internet]. 1989 Oct [cited 2015 Jun 1];29(5):699–702.A
114. McMinn BG, Hinton L. Confined to barracks: The effects of indoor confinement on aggressive behavior among inpatients of an acute psychogeriatric unit. Am J Alzheimers Dis Other Demen [Internet]. 2000 Jan 1 [cited 2015 Jun 1];15(1):36–41.
115. Namazi K, Johnson B. Pertinent autonomy for residents with dementias: Modification of the physical environment to enhance independence. Am J Alzheimers Dis Other Demen. 1992;7:16–21.
116. Vitiello M V., Borson S. Sleep Disturbances in Patients with Alzheimer??s Disease. CNS Drugs [Internet]. 2001 [cited 2015 Jun 10];15(10):777–96.
117. Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatr Scand [Internet]. 1994 Jan [cited 2015 Jun 1];89(1):1–7.
118. Okawa M, Mishima K, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep [Internet]. 1991 Dec [cited 2015 Jun 1];14(6):478–85.
119. Lovell BB, Ancoli-Israel S, Gevirtz R. Effect of bright light treatment on agitated behavior in institutionalized elderly subjects. Psychiatry Res [Internet]. 1995 Jun 29 [cited 2015 Jun 1];57(1):7–12.
120. Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry [Internet]. 1999 Jul [cited 2015 Jun 1];14(7):520–5.
121. Koss E, Gilmore GC. Environmental interventions and functionability of AD patients. In: Vellas B, Fitten J, Frisoni G, editors. Research and Practice in Alzheimer’s Disease. Paris/New York: Serdi/Springe; 1998. p. 185–191.
122. Thorpe L, Middleton J, Russell G, Stewart N. Bright light therapy for demented nursing home patients with behavioral disturbance. Am J Alzheimers Dis Other Demen [Internet]. 2000 Jan 1 [cited 2015 Jun 1];15(1):18–26.
123. Skjerve A, Bjorvatn B, Holsten F. Light therapy for behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry [Internet]. 2004 Jun [cited 2015 Jun 10];19(6):516–22.
124. Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane database Syst Rev [Internet]. 2014 Jan [cited 2016 Jan 21];2:CD003946.
125. McCallion P, Toseland RW, Lacey D, Banks S. Educating nursing assistants to communicate more effectively with nursing home residents with dementia. Gerontologist [Internet]. 1999 Oct [cited 2015 Jun 1];39(5):546–58.
126. Williams DP. An In-Service Workshop for Nursing Personnel on the Management of Catastrophic Reactions in Dementia Victims. Clin Gerontol [Internet]. Taylor & Francis Group; 1994 Jun 3 [cited 2015 Jun 1];14(4):47–54. A
127. Wells DL, Dawson P, Sidani S, Craig D, Pringle D. Effects of an abilities-focused program of morning care on residents who have dementia and on caregivers. J Am Geriatr Soc [Internet]. 2000 Apr [cited 2015 Apr 27];48(4):442–9.
128. Mentes JC, Ferrario J. Calming aggressive reactions–a preventive program. J Gerontol Nurs [Internet]. 1989 Feb [cited 2015 Apr 14];15(2):22–7.
129. Matteson MA, Linton AD, Cleary BL, Barnes SJ, Lichtenstein MJ. Management of problematic behavioral symptoms associated with dementia: a cognitive developmental approach. Aging (Milano) [Internet]. 1997 Oct [cited 2015 Jun 1];9(5):342–55.
130. Hébert R, Lévesque L, Vézina J, Lavoie J-P, Ducharme F, Gendron C, et al. Efficacy of a psychoeducative group program for caregivers of demented persons living at home: a randomized controlled trial. J Gerontol B Psychol Sci Soc Sci [Internet]. 2003 Jan [cited 2015 Jun 1];58(1):S58-67. A
131. Eloniemi-Sulkava U, Notkola IL, Hentinen M, Kivelä SL, Sivenius J, Sulkava R. Effects of supporting community-living demented patients and their caregivers: a randomized trial. J Am Geriatr Soc [Internet]. 2001 Oct [cited 2015 Jun 1];49(10):1282–7.
132. Mittelman MS, Ferris SH, Shulman E, Steinberg G, Levin B. A family intervention to delay nursing home placement of patients with Alzheimer disease. A randomized controlled trial. JAMA [Internet]. 1996 Dec 4 [cited 2015 Jun 1];276(21):1725–31.
133. Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry [Internet]. 2005 Nov [cited 2015 Jun 1];162(11):1996–2021.
134. Aronstein Z, Olsen R, Schulman E. the nursing assistant’s use of recreational interventions for behaviour management of residents with Alzheimer’s disease. Am J Alzheimer’ care Relat Disord Res. 1996;11:26–31.
135. Groene RW. Effectiveness of Music Therapy 1:1 Intervention with Individuals Having Senile Dementia of the Alzheimer’s Type. J Music Ther [Internet]. Oxford University Press; 1993 Sep 1 [cited 2015 Jun 1];30(3):138–57.
136. Kolanowski A, Fick DM, Buettner L. Recreational Activities to Reduce Behavioural Symptoms in Dementia. Geriatr Aging [Internet]. 2009 Jan 1 [cited 2015 Oct 5];12(1):37–42.
137. Calkins M, Szmerekovsky JG, Biddle S. Effect of Increased Time Spent Outdoors on Individuals with Dementia Residing in Nursing Homes. J Hous Elderly [Internet]. 2007 Dec 18 [cited 2015 Jun 10];21(3–4):211–28.
138. Detweiler MB, Murphy PF, Myers LC, Kim KY. Does a wander garden influence inappropriate behaviors in dementia residents? Am J Alzheimers Dis Other Demen [Internet]. 2008 Jan [cited 2015 Jun 10];23(1):31–45.
139. Tadros G, Ormerod S, Dobson-Smyth P, Gallon M, Doherty D, Carryer A, et al. The management of behavioural and psychological symptoms of dementia in residential homes: does Tai Chi have any role for people with dementia? Dementia [Internet]. 2013 Mar 20 [cited 2015 May 26];12(2):268–79.
140. Steinberg M, Leoutsakos J-MS, Podewils LJ, Lyketsos CG. Evaluation of a home-based exercise program in the treatment of Alzheimer’s disease: the Maximizing Independence in Dementia (MIND) study. Int J Geriatr Psychiatry [Internet]. 2009 Jul [cited 2014 May 25];24(7):680–5.
141. Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil [Internet]. 2004 Oct [cited 2014 Jul 4];85(10):1694–704.